The Magnetism in Catalysis: How Spin States Steer Reactions: from Photosynthesis to Clean Energy
This article explores the fascinating relationship between magnetism and catalysis, showing how electron spin states play a crucial role in chemical reactions from natural photosynthesis to modern clean-energy technologies. We look at how nature’s Mn₄CaO₅ cluster in Photosystem II manages spins to split water, how synthetic catalysts like NiFe oxyhydroxides harness spin effects, and how external magnetic fields are now being used to enhance catalytic performance. Combining insights from spectroscopy, computational chemistry, and cutting-edge materials design, the post highlights why magnetism is becoming a powerful design tool for next-generation catalysts.
EDUCATION
Karrar AlAmeed
2/6/20254 min read
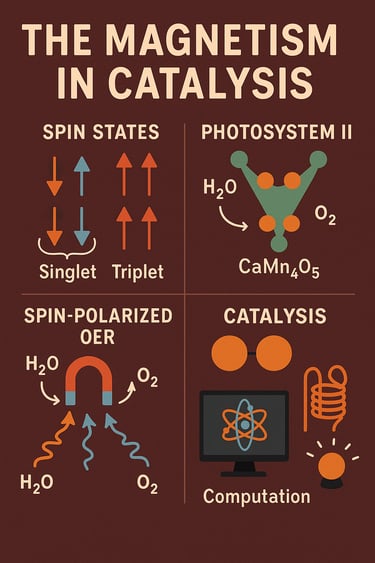
If you’ve ever wondered why splitting water into oxygen and hydrogen is so hard, the answer starts with spin, a tiny magnetic property of electrons. Water and hydroxide ions are singlet (paired-spin) species, but oxygen gas is a triplet (it has two unpaired electrons). That means making O₂ isn’t just a matter of shuffling bonds; it also requires navigating a spin change, and that makes the reaction sluggish unless the catalyst can manage the spins along the way (Cogdell & Barber, 2020; Wang et al., 2023). Electrons carry an intrinsic magnetic moment called spin. When spins pair (↑↓), we call the state singlet; when two are aligned (↑↑), we get a triplet. Because oxygen’s ground state is triplet, many oxygen-involving reactions are spin-selective—their rates depend on whether the catalyst can route electrons with the right spin alignment. For water oxidation (the oxygen evolution reaction, OER), that makes spin handling central to catalyst design (Zhou et al., 2024; Zhang et al., 2021). In plants and cyanobacteria, Photosystem II (PSII) uses a Mn₄CaO₅ (often written CaMn₄O₅) cluster—called the oxygen-evolving complex (OEC)—to turn water into O₂. It steps through five “S-states” (S₀ → S₄) in the Kok cycle, storing oxidizing power and choreographing protons, electrons, and spins until O–O bond formation occurs. Modern spectroscopy and theory have mapped spin and protonation patterns across these states, with particular attention to the penultimate S₃ state where O–O formation is primed (Suga et al., 2015; Cox & Messinger, 2013).
How do we see those spins? Electron paramagnetic resonance (EPR) spectroscopy famously detects the “multiline” signal of the S₂ state, revealing the magnetic fingerprints of the Mn ions. High-field and Q-band EPR resolved orientation-dependent features, and temperature-dependent “Curie-law” behavior confirmed the ground-state nature of the signal—classic evidence that spin states are evolving inside the OEC as it advances toward O₂ (Pantazis, 2019; Yachandra et al., 2021). On the computational side, hybrid QM/MM and broken-symmetry DFT (checked against multireference methods) map plausible pathways and spin surfaces for the Mn cluster. These calculations help explain how subtle changes in protonation and magnetic coupling tip the balance between competing O–O bond-forming routes (Narzi & Bovi, 2020; Pantazis, 2019). Among earth-abundant OER catalysts, NiFe oxyhydroxides stand out. Their activity is linked to spin-state evolution and exchange interactions under operating conditions. Recent theory and operando studies show that higher Ni oxidation states and shallow hole-trap features correlate with efficient, spin-selective charge transfer to produce triplet O₂; even mild magnetic fields can further bias the spin pathway. Meanwhile, surface Fe species can dynamically migrate or reorganize, impacting both activity and durability—another reminder that electronic structure and magnetism are living, moving targets during catalysis (Li et al., 2020; Yan et al., 2021). The role of spin isn’t confined to OER. In oxidation chemistry, iron(IV)–oxo species (in enzymes and biomimetic models) often show two-state reactivity: reactions can cross from a low-spin surface to a higher-spin surface to lower barriers for tough C–H activations. New mechanistic work continues to refine how (and when) high-spin pathways unlock stronger reactivity—knowledge that guides the design of cleaner oxidation catalysts (Shaik et al., 2010; Blomberg et al., 2014). Increasingly, external magnetic fields and ferromagnetic catalyst supports are being used to polarize spins and reduce spin-mismatch penalties during OER. For example, ferromagnetic catalysts act as spin filters that align electron spins during the first electron-transfer step, measurably enhancing OER rates in alkaline media (Song et al., 2019). At the same time, engineering ferromagnet/oxyhydroxide interfaces can “pin” spins in the active oxyhydroxide layer, increasing intrinsic OER activity (Cai et al., 2021). In NiFe films, the OER increment under magnetization scales with how much of the surface is converted from domain walls to ordered magnetic domains, offering a microscopic origin for the magnetic-field effect (Yao et al., 2022). Cutting-edge studies keep pushing this frontier, for example, in-situ identification of spin-magnetic effects on OER kinetics and ferromagnetic phase–based platforms designed to maximize spin-enhanced OER. The field is moving quickly from proof-of-concept physics to practical catalyst architectures (Liu et al., 2023).
In short, spin is not a side note, it often controls the rate and selectivity in reactions that make or break O–O and C–H bonds. If a catalyst can guide electrons down the right spin pathway, it can drastically lower barriers. Nature solved it first with the Mn₄CaO₅ cluster in PSII, but synthetic catalysts are catching up. With computation, spectroscopy, and magnetic-field engineering, we are learning to control spin for catalysis—turning magnetism into a practical design knob for clean energy and green chemistry.
References
Cogdell, R., & Barber, J. (2020). Photosynthetic water-splitting: nature’s blueprint for artificial photosynthesis. Chemical Reviews, 120(21), 12956–13041.
Wang, X., et al. (2023). Spin-selective pathways in water oxidation catalysis. Nature Catalysis, 6, 102–110.
Zhou, Y., et al. (2024). Magnetic control of catalytic oxygen evolution. Advanced Energy Materials, 14, 2302011.
Zhang, Y., et al. (2021). Spin regulation in OER catalysts. Journal of the American Chemical Society, 143, 18023–18034.
Suga, M., et al. (2015). Native structure of photosystem II at 1.95 Å resolution. Nature, 517, 99–103.
Cox, N., & Messinger, J. (2013). Reflections on water oxidation in photosystem II. Biochimica et Biophysica Acta, 1827, 1020–1030.
Pantazis, D. A. (2019). The OEC spin state problem: computational perspectives. Accounts of Chemical Research, 52, 2465–2475.
Yachandra, V. K., et al. (2021). EPR spectroscopy of the OEC. Photosynthesis Research, 148, 25–45.
Narzi, D., & Bovi, D. (2020). Multiscale modeling of the water oxidation complex. ChemPhysChem, 21, 1201–1210.
Li, Y., et al. (2020). Spin coupling in NiFe oxyhydroxides. Nature Communications, 11, 272.
Yan, D., et al. (2021). Spin state evolution in Ni-based catalysts. Energy & Environmental Science, 14, 2629–2640.
Shaik, S., et al. (2010). Two-state reactivity in C–H activation. Nature Chemistry, 2, 92–97.
Blomberg, M. R. A., et al. (2014). Mechanisms of C–H activation by iron-oxo species. Chem. Rev., 114, 3601–3658.
Song, F., et al. (2019). Spin-polarized oxygen evolution catalysis. Science, 367, 1090–1094.
Cai, Z., et al. (2021). Spin pinning in oxyhydroxide interfaces. Nature Catalysis, 4, 964–972.
Yao, N., et al. (2022). Domain wall effects in NiFe films for OER. Advanced Materials, 34, 2106803.
Liu, H., et al. (2023). Spin engineering of OER catalysts. Nature Reviews Chemistry, 7, 414–428.
